Anal cancer is on the rise, especially in women. Should they be screened?
Currently, there are no accepted screening guidelines. The average woman has a tiny lifetime risk of anal cancer (two in 100,000), so figuring out which women to check, and the best way to do it, is full of dilemmas.

Now that actress Marcia Cross has been in remission from anal cancer for about a year, she has a mission.
“I want to help put a dent in the stigma around anal cancer,” the former Desperate Housewives star, 57, recently told People magazine. “I’ve read a lot of cancer survivor stories and many people — women, especially — were too embarrassed to say what kind of cancer they had. There is a lot of shame about it. I want it to stop.”
Cross, who was diagnosed in November 2017, is not the first celebrity to say we need to get over our squeamishness. Actress Farrah Fawcett made a documentary about her harrowing struggle before anal cancer ended her life in 2009.
But Cross is speaking out at an opportune juncture. While cancer of the anus – the short canal at the end of the rectum through which solid waste leaves the body – remains fairly rare, it has been steadily increasing for several decades, and far more women than men develop it. The American Cancer Society estimates that about 5,530 women and 2,770 men will be diagnosed this year; about 760 women and 520 men will die.
In response to this alarming trend, leading experts — specialists in oncology, pathology, gynecology, infectious diseases, and more — believe it’s time to start screening some women for anal cancer.
There are no accepted screening guidelines because, well, it’s complicated. The average woman has a tiny lifetime risk of anal cancer (two in 100,000), so figuring out which women to check, and the best way to do it, is full of dilemmas, as the experts explained in their 2016 paper, Screening for Anal Cancer in Women.
“We don’t want to spend a lot of testing resources, and create a lot of anxiety, in low-risk groups of women,” said senior author Joel M. Palefsky, an infectious disease physician and anal cancer expert at the University of California-San Francisco.
But there is something that low-risk women can do to be vigilant.
Mysterious vulnerabilities
To better understand why screening all women doesn’t make sense, consider the cause of 90 percent of anal cancers: the human papillomavirus (HPV).
HPV is a ubiquitous family of sexually transmitted viruses that live in cells on the surface of the anus, cervix, vagina, vulva, penis, mouth, and throat. Although the immune system wipes out most infections with nary a symptom, persistent infection with high-risk HPV types can initiate cancer in those organs.
It should be noted that the Gardasil vaccine, recommended to be given in adolescence, prevents infection with nine high-risk types, as well as two types that cause genital warts. But the immunization remains underused – and it wasn’t available until 2006.
Where Anal Cancer Develops
The anal canal is a 2-inch-long tube below the rectum. Stool leaves the body through the anus, opening at the end of the canal.
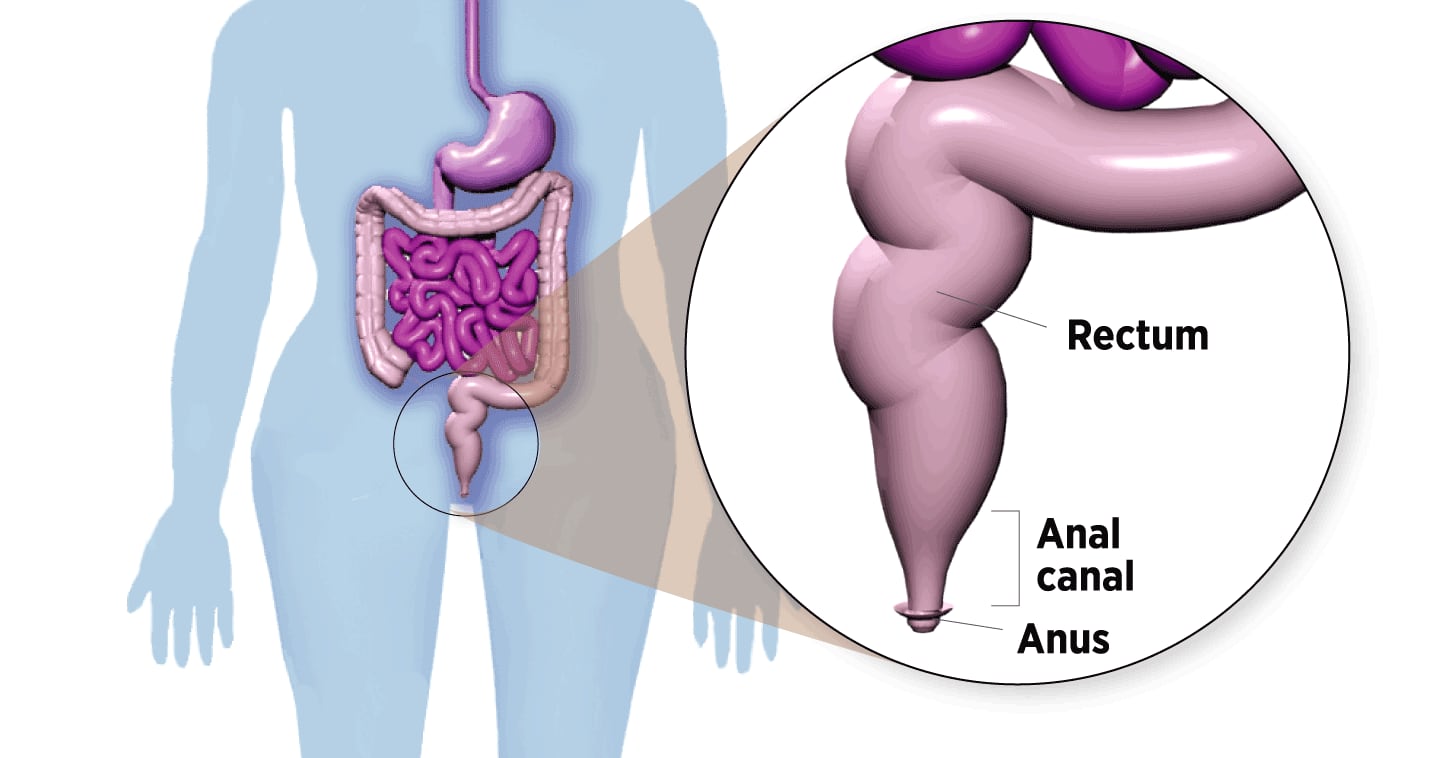
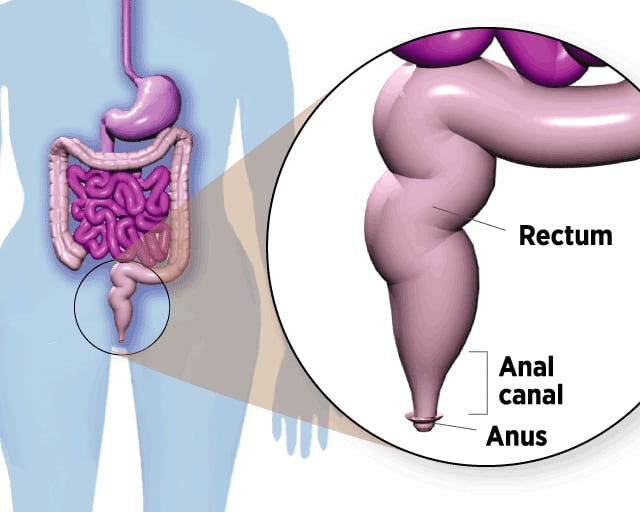

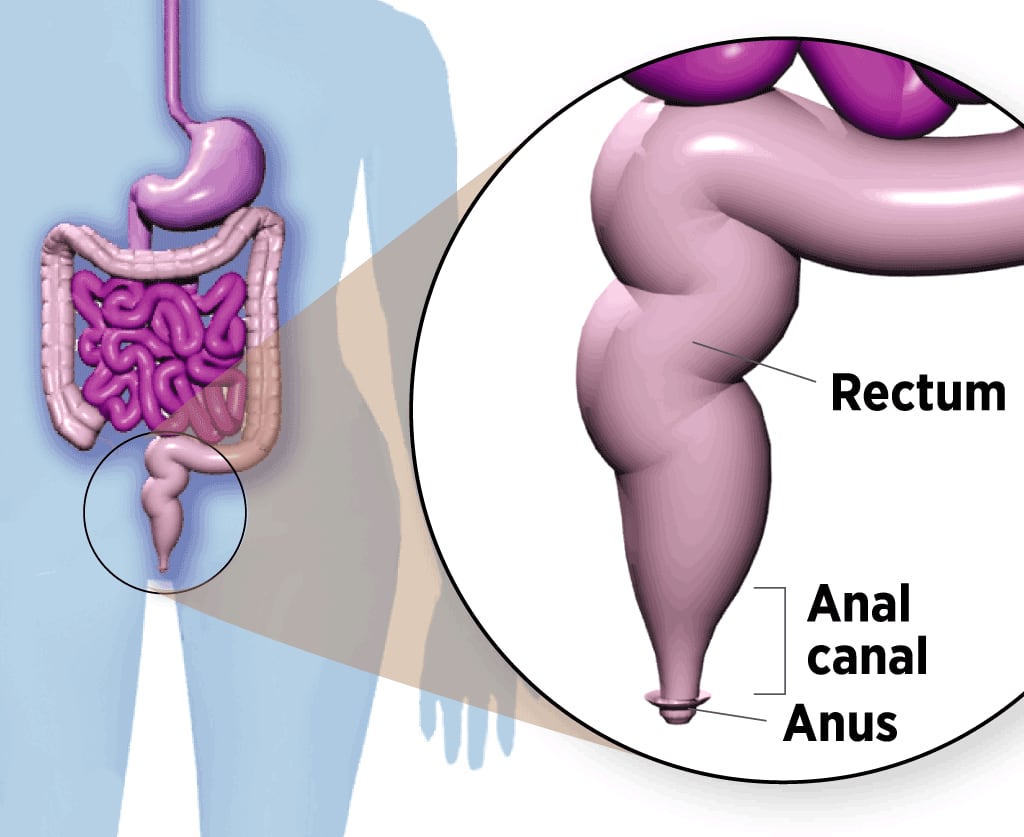
In theory, anal cancer could be prevented before it develops, just like cervical cancer. Both diseases start with precancerous lesions that gradually progress to malignancy. By taking a swab of anal cells and checking them under the microscope – the anus version of the cervical Pap smear – a worrisome abnormality could be detected early and removed.
The low-tech Pap smear, introduced in the 1950s, transformed cervical cancer from the leading cancer killer of U.S. women to an uncommon threat. These days, a DNA test that detects high-risk HPV infection can be used instead of the smear. (A few anal cancer screening studies of gay men have used the DNA test to verify results of anal smear tests.)
The tricky part is, HPV does not behave the same in all tissues. For reasons that remain unclear, the cervix is highly likely to develop HPV-driven precancers. The anus is not — even though studies have found that healthy women have high rates of anal HPV.
The lead author of the screening paper, Anna-Barbara Moscicki, a pediatrician and HPV researcher at the University of California-Los Angeles, said the rarity of anal lesions is only partly explained by immune responses and sexual practices.
“Why is the cervix so much more vulnerable than the anus? We don’t know,” she said.
Defining ‘high-risk’
To prove the theory that screening with anal smears can detect precancers and thus reduce cancers, Palefsky, at UCSF, is leading a government-funded clinical trial called ANCHOR. When lesions are found in the women and men in the trial, they are randomly assigned to have the lesions either treated or monitored every six months. Both groups are being followed for five years.
The trial is limited to people known to be at very high risk of anal cancer — namely, those infected with HIV, the AIDS virus. That virus compromises their immune systems, so they are prone to HPV infection. One study found more than a quarter of HIV-positive women had severe anal precancers.
Another group that the experts recommend screening for anal cancer is organ transplant recipients, who take immune-suppressing drugs to prevent organ rejection.
A much bigger group – women who have been treated for cervical precancers or cancer, or vulvar cancer – might benefit from screening, the experts said. There aren’t enough data to know.
“They’re at higher risk, but we don’t know how high,” Palefsky said.
Another issue raised by screening involves the availability of high-resolution anoscopy, the gold standard procedure for diagnosing abnormalities found on an anal smear. During a 15-minute office procedure, done without any bowel preparation, the clinician uses a thin cylindrical scope to examine the anal canal. If needed, a tissue sample, or biopsy, can be taken for lab analysis. Anoscopy provides much greater tissue magnification than colonoscopy, used to screen for colorectal cancer.
But not many people have been trained to do anoscopies, at least not yet.
“There are about 50 clinics in the U.S. that do the procedure,” said Naomi Jay, a San Francisco nurse practitioner who performs and teaches it. She is also president of the seven-year-old International Anal Neoplasia Society, which helped convene the experts who wrote the screening paper.
The final screening quandary may be the thorniest. Even though the average woman has minuscule risk of anal cancer, “this group of healthy women make up the largest percent of women who develop" it, the experts wrote.
For them, the screening fallback is the digital anorectal exam, during which a clinician feels the anal canal with a finger.
A digital exam won’t detect a precancer, and the effectiveness depends on the skill of the clinician. (It is not the same as the digital exam done as part of a pelvic exam.)
But it made all the difference for Marcia Cross.
The actress — who had no anal cancer symptoms such as bleeding or pain — said her gynecologist felt her tumor at a relatively early stage by doing a digital anorectal exam during a routine checkup. Chemotherapy and radiation were “gnarly,” but successful, she said.
“Marcia was lucky because she had a gynecologist who did the exam,” Jay said. “Minimally, every woman deserves to have a digital anorectal exam.”