Top health official says he was ousted over Trump-touted drug
A top U.S. health official who was helping lead efforts to find a coronavirus vaccine said he was removed from his post because he insisted on limiting the use of a drug President Donald Trump has pushed as a COVID-19 treatment despite little clinical evidence it works.
WASHINGTON — A top U.S. health official who was helping lead efforts to find a coronavirus vaccine said he was removed from his post because he insisted on limiting the use of a drug President Donald Trump has pushed as a COVID-19 treatment despite little clinical evidence it works.
Rick Bright was abruptly pushed out of his position as the director of the Biomedical Advanced Research and Development Authority on Tuesday and given a smaller role at the National Institutes of Health. BARDA is helping pharmaceutical companies develop a vaccine for the novel coronavirus.
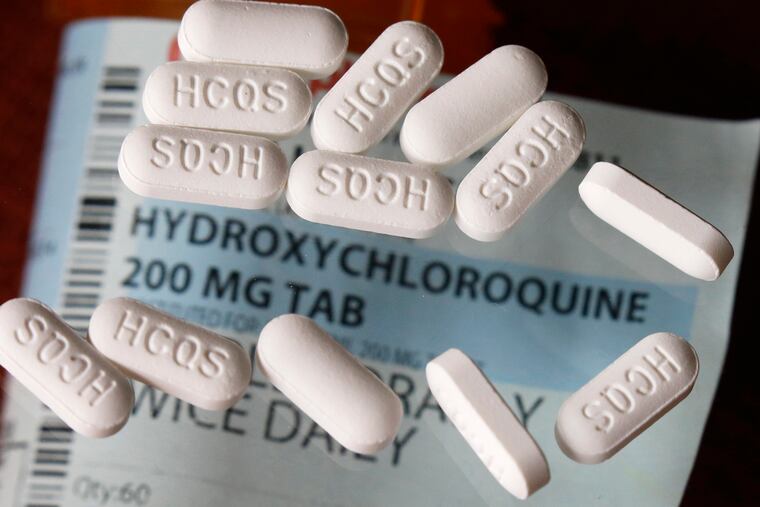
The drugs Trump has touted, hydroxychloroquine and chloroquine, “clearly lack scientific merit,” Bright said in a statement through his lawyers on Wednesday.
“While I am prepared to look at all options and to think ‘outside the box’ for effective treatments, I rightly resisted efforts to provide an unproven drug on demand to the American public,” Bright said. “I insisted that these drugs be provided only to hospitalized patients with confirmed COVID-19 while under the supervision of a physician. These drugs have potentially serious risks associated with them, including increased mortality observed in some recent studies in patients with COVID-19.”
Bright’s former agency received an emergency authorization last month from the Food and Drug Administration to use hydroxychloroquine and chloroquine in the national stockpile and dole the drugs out for hospitalized COVID-19 patients. BARDA oversees the stockpile.
During an April 10 interview with Bloomberg Businessweek, Bright emphasized the need for conducting rigorous scientific research even in the midst of a pandemic.
» FAQ: Your coronavirus questions, answered
“It is very difficult to do development and conduct research in the middle of a pandemic outbreak,” he said during a phone call that focused on some of BARDA’s drug research in COVID-19 treatments.
“Everyone wants to grab onto any idea, any anecdotal data or any observational data, but what we are really trying to do is conduct randomized controlled trials to get high quality data,” Bright said.
Randomized controlled trials are the gold-standard studies in which some patients are randomly assigned a placebo or standard treatment, in order to provide a unbiased comparison of whether an experimental medicine being studied works.
While his agency was looking at both off-the-shelf and custom drugs to combat the coronavirus, Bright emphasized that none had been proven to work.
“We really don’t have any drug right now with clear data on its benefit and impact on this coronavirus,” he said.
Less than two weeks later, he was out of his job.
Bright’s lawyers at Katz, Marshall & Banks have asked that he be allowed to remain in his top post at BARDA pending government investigations into his removal.
“Sidelining me in the middle of this pandemic and placing politics and cronyism ahead of science puts lives at risk and stunts national efforts to safely and effectively address this urgent public health crisis,” Bright said in his statement.