CDC endorses Pfizer COVID-19 shot for kids 12 and up
The sprint to vaccinate millions of middle and high school students has already started in parts of the country.
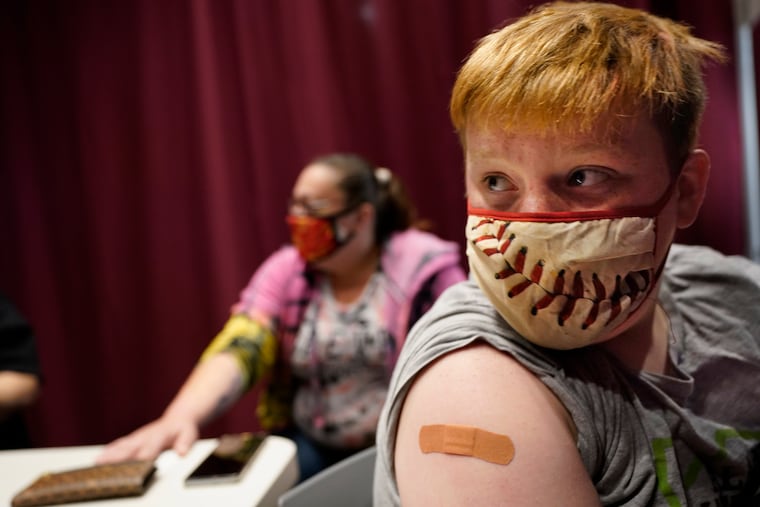
U.S. health advisers endorsed use of Pfizer’s COVID-19 vaccine in kids as young as 12 on Wednesday — just as planned new guidelines say it’s OK for people of any age to get a coronavirus shot at the same time as other needed vaccinations.
The shots will let kids safely attend camps this summer and help assure a more normal return to classrooms next school year, concluded advisers to the Centers for Disease Control and Prevention.
“And this is another way to get closer to ending this horrible pandemic,” said adviser Dr. Camille Kotton of Harvard Medical School.
The sprint to vaccinate millions of middle and high school students has already started in parts of the country, as a long line of kids rolled up their sleeves in suburban Atlanta for a first dose Wednesday.
“It just felt like a flu shot, honestly,” said Meredith Rogers, 14, from Decatur, Georgia, after getting her vaccination.
Michelle Rogers, Meredith’s mother, said she hoped the youth vaccinations would help bring some normalcy back.
“A little apprehensive, but you know what? This is a step towards getting life back to normal so, we’re all in,” Michelle Rogers said with a slight fist pump.
Pfizer’s vaccine has been used for months in people 16 and older, and earlier this week the Food and Drug Administration cleared its use for those as young as age 12. But before rolling it out to the younger kids, much of the nation was awaiting recommendations from CDC’s advisers — and the panel concluded the same dose adults use is safe and strongly protective in those 12 to 15 years old, too.
The CDC rapidly accepted its advisers’ recommendation.
A key question: Is it OK to get vaccinated against COVID-19 at the same doctor’s visit as people receive some routine vaccinations? That’s an urgent back-to-school concern especially for the 12- to 15-year-olds, who have missed out on regularly scheduled vaccines during the pandemic — but it’s an issue for adults, too.
The CDC until now has recommended not getting other vaccinations within two weeks of a COVID-19 shot, mostly as a precaution so that safety monitors could spot if any unexpected side effects cropped up.
But the CDC said Wednesday it is changing that advice because the COVID-19 vaccines have proved very safe — and that health workers can decide to give another needed vaccine at the same time for people of any age.
“The need for catch-up vaccination in coordination with COVID-19 vaccination is urgent as we plan for safe return to school,” CDC’s Dr. Kate Woodworth told the panel, citing millions of missed doses of vaccines against tetanus, whooping cough and other health threats.
The American Academy of Pediatrics on Wednesday also urged that kids 12 and older get the Pfizer vaccine — and agreed that it’s fine to give more than one vaccine at the same time, especially for kids who are behind on their regular vaccinations.
Children are far less likely than adults to get seriously ill from COVID-19 -- but they do sometimes die, and thousands have been hospitalized. By last month, those ages 12 to 17 were making up slightly more of the nation’s new coronavirus infections than adults over 65, a group that’s now largely vaccinated.
The two-dose vaccine made by Pfizer and its German partner BioNTech was studied in more than 2,000 kids ages 12 to 15. There were no cases of COVID-19 among vaccinated kids compared with 16 in the group given dummy shots. Kids also developed higher levels of virus-fighting antibodies than vaccinated adults.
Side effects are the same as adults experience, mostly sore arms and flu-like fever, chills or aches that signal the immune system is revving up.
CDC’s advisers did caution that those temporary shot reactions may be even more common if people get a COVID-19 shot at the same time as another vaccination.
President Joe Biden hailed Wednesday’s vote, noting that means 17 million more people in the U.S. now qualify to get vaccinated.
“I encourage their parents to make sure they get the shot,” he said. “As I promised last week, we’re ready. This new population is going to find the vaccine rollout fast and efficient.”
In addition to the mass vaccination sites and health department rollouts that were key for adults, many states will be offering kids more familiar options -- shipping doses to pediatricians and even to schools.
Pfizer is not the only company seeking to lower the age limit for its vaccine. Moderna recently said preliminary results from its study in 12- to 17-year-olds show strong protection and no serious side effects, data the FDA will need to scrutinize.
As for even younger children, both companies have begun tests in youngsters ages 6 months to 11 years. Those studies explore if different doses are needed at the youngest ages, and FDA plans to hold a public meeting next month to debate exactly what evidence is needed.