CDC panel will meet Tuesday to vote on COVID-19 vaccine priority
A panel of U.S. advisers will meet next week to recommend who should be first in line for a COVID-19 vaccine
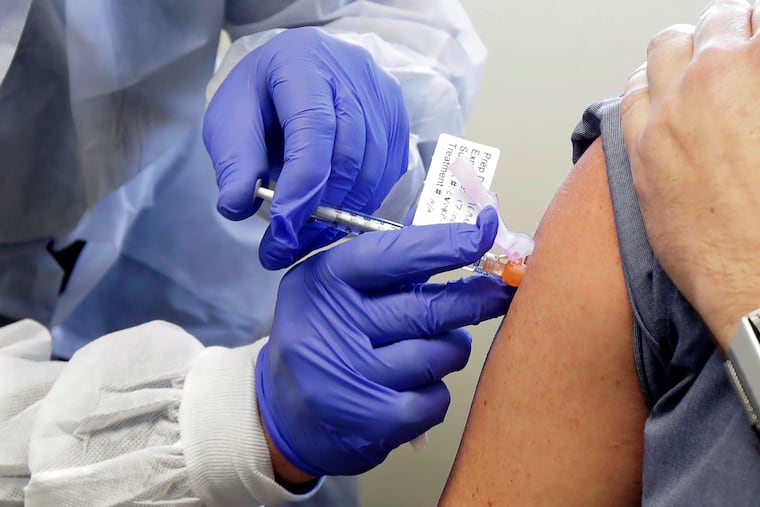
ATLANTA — A panel of U.S. advisers will meet Tuesday to vote on how scarce, initial supplies of a COVID-19 vaccine will be given out once one has been approved.
Experts have proposed giving the vaccine to health workers first. High priority also may be given to workers in essential industries, people with certain medical conditions and people age 65 and older.
Tuesday's meeting is for the Advisory Committee on Immunization Practices, a group established by the Centers for Disease Control and Prevention. The panel of experts recommends who to vaccinate and when -- advice that the government almost always follows. The agenda for next week's emergency meeting was posted Friday.
Pfizer and its German partner BioNTech have asked the Food and Drug Administration to allow emergency use of its COVID-19 vaccine candidate. Moderna Inc. is expected to also seek emergency use of its vaccine soon.
FDA's scientific advisers are holding a public meeting Dec. 10 to review Pfizer's request, and send a recommendation to the FDA.
Manufacturers already have begun stockpiling coronavirus vaccine doses in anticipation of eventual approval, but the first shots will be in short supply and rationed.