Pfizer’s COVID antiviral pill, Paxlovid, is becoming more widely available. Here’s what to know.
Explaining Pfizer's antiviral COVID-19 drug, Paxlovid: How it works, side effects, and who can get it.
The White House is working to expand availability of an antiviral COVID-19 treatment known as Paxlovid, President Joe Biden’s administration announced Tuesday. Produced by Pfizer, the drug is intended to help prevent those at the highest risk from developing severe illness.
Efforts by the Biden administration include physician outreach, direct distribution of the drug to pharmacies, and purchasing a supply of the treatment that should last the nation several months. Initially, the drug was limited in supply, but manufacturing has since increased, and it is now far more available, the Associated Press reports.
“The bottom line is that we want to make this therapeutic available to all Americans,” White House COVID-19 response coordinator Ashish Jha said Tuesday on CNN.
» READ MORE: The Biden administration is expanding the availability of COVID-19 antiviral pill Paxlovid
So, what exactly is Paxlovid, and how does it work? Are there any side effects you should consider before taking it? Here is what you need to know:
What is Paxlovid?
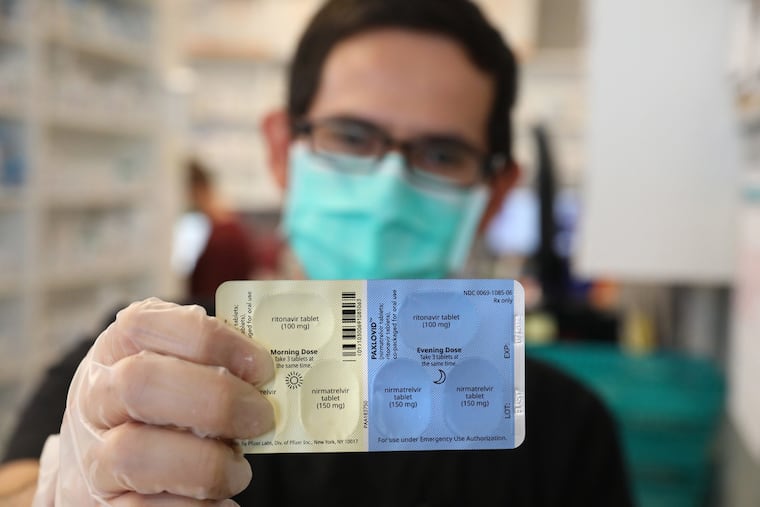
Pfizer’s Paxlovid is an antiviral drug developed to treat COVID-19. It received an emergency use authorization from the Food and Drug Administration in December.
When administered within five days of the onset of symptoms, it is an effective treatment. According to the FDA, Paxlovid has been proven to reduce hospitalizations and deaths among patients at high risk for developing severe disease by nearly 90%.
The World Health Organization this month issued a strong statement of support for the drug, calling it “the best therapeutic choice for high-risk patients to date.”
How does Paxlovid work?
Paxlovid is a combination of two drugs — nirmatrelvir and ritonavir — taken together orally, twice daily for five days. Nirmatrelvir works to inhibit the coronavirus’ ability to replicate, and ritonavir slows the breakdown of nirmatrelvir, allowing higher concentrations of the drug to stay in the body for a longer period.
In total, a full course of treatment consists of 30 tablets, with each dose being two tablets of nirmatrelvir and one tablet of ritonavir. Paxlovid, the FDA said in December, is not authorized for use for more than five consecutive days.
What are Paxlovid’s side effects?
For the most part, potential side effects from taking Paxlovid are relatively mild and can include muscle aches, high blood pressure, diarrhea, and impaired sense of taste. But for some people, such as those with uncontrolled or undiagnosed HIV infections or preexisting liver diseases, side effects can be more severe, so the FDA recommends they exercise caution in taking the drug.
Paxlovid can also have adverse interactions with a number of other drugs, primarily because it works by inhibiting enzymes in the body that help break them down. As The Inquirer reported in February, contraindicated drugs include common cardiac medications like Plavix and amiodarone, along with some anticancer, antipsychotic, and analgesic drugs — as well as herbal treatments like St. John’s wort, manufacturer Pfizer notes online.
Who can get Paxlovid?
Paxlovid is approved for use by adults and children age 12 and up who have tested positive for COVID-19 and are at high risk for developing severe disease. That includes people with conditions such as asthma, heart disease, and diabetes, or those who are overweight or obese. However, because of the way the drug works, Paxlovid is not recommended for people with serious liver or kidney problems, according to the FDA fact sheet.
How can you get Paxlovid?
If you believe you are eligible for a prescription, you can ask your doctor about being prescribed the drug. The White House said it is increasing doctor outreach efforts and encouraging medical professionals to prescribe Paxlovid to eligible patients. The drug is also now being directly distributed to pharmacies and should be available at more than 30,000 locations nationwide by next week.
You may also be able to use the federal government’s test-to-treat database to find locations that can test for COVID-19 and prescribe Paxlovid on-site. At the moment, there are 2,200 such locations around the country, but the Biden administration hopes to add to that number in the coming weeks, the Associated Press reports.
How much does Paxlovid cost?
The Biden administration has secured 20 million treatment courses of the drug at a reported cost of about $530 each. Those courses will be distributed free of charge, so eligible patients shouldn’t have to pay anything for the medication itself.