FDA clears Pfizer COVID-19 vaccinations in young kids
The FDA cleared kid-size doses for emergency use, and up to 28 million more American children could be eligible for vaccinations.
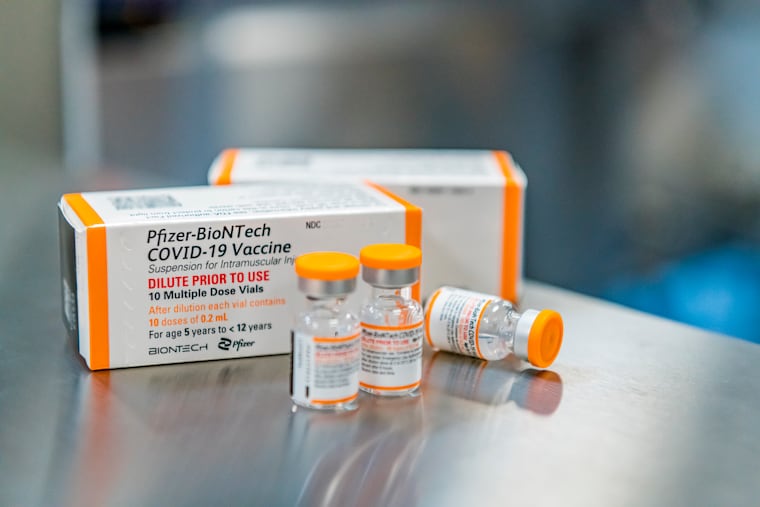
WASHINGTON — The Food and Drug Administration on Friday paved the way for children ages 5 to 11 to get Pfizer’s COVID-19 vaccine.
The FDA cleared kid-size doses — just a third of the amount given to teens and adults — for emergency use, and up to 28 million more American children could be eligible for vaccinations as early as next week.
One more regulatory hurdle remains: On Tuesday, advisers to the Centers for Disease Control and Prevention will make more detailed recommendations on which youngsters should get vaccinated, with a final decision by the agency’s director expected shortly afterwards.
“The rationale here is protect your children so that they can get back towards normal life,” said FDA vaccine chief Dr. Peter Marks. “The tremendous cost of this pandemic has not just been in physical illness, it’s been in the psychological, the social development of children” too.
A few countries have begun using other COVID-19 vaccines in children under 12, including China, which just began vaccinations for 3-year-olds. But many that use the vaccine made by Pfizer and its partner BioNTech are watching the U.S. decision, and European regulators just began considering the companies’ kid-size doses.
With FDA’s action, Pfizer plans to begin shipping millions of vials of the pediatric vaccine — in orange caps to avoid mix-ups with the purple-capped doses for everyone else — to doctors’ offices, pharmacies and other vaccination sites. Once the CDC issues its ruling, eligible kids will get two shots, three weeks apart.
While children are at lower risk of severe illness or death from COVID-19 than older people, 5- to 11-year-olds still have been seriously affected -- including over 8,300 hospitalizations, about a third requiring intensive care. The FDA said 146 deaths have been reported in that age group.
And with the extra-contagious delta variant circulating, the government has counted more than 2,000 coronavirus-related school closings just since the start of the school year, affecting more than a million children.
“With this vaccine kids can go back to something that’s better than being locked at home on remote schooling, not being able to see their friends,” said Dr. Kawsar Talaat of Johns Hopkins University. “The vaccine will protect them and also protect our communities.”
The American Academy of Pediatrics also applauded FDA’s decision, and said pediatricians were “standing by” to talk with parents.
Vaccinating this age group is “an important step in keeping them healthy and providing their families with peace of mind,’’ said Dr. Lee Savio Beers, the academy’s president.
Earlier this week, FDA’s independent scientific advisers voted that the pediatric vaccine’s promised benefits outweigh any risks. But several panelists said not all youngsters will need to be vaccinated, and that they preferred the shots be targeted to those at higher risk from the virus.
Nearly 70% of 5- to 11-year-olds hospitalized for COVID-19 in the U.S. have other serious medical conditions, including asthma and obesity, according to federal tracking. Additionally, more than two-thirds of youngsters hospitalized are Black or Hispanic, mirroring long-standing disparities in the disease’s impact.
The question of how broadly Pfizer’s vaccine should be used will be a key consideration for the CDC and its advisers, who set formal recommendations for pediatricians and other medical professionals.
A Pfizer study of 2,268 schoolchildren found the vaccine was nearly 91% effective at preventing symptomatic COVID-19 infections, based on 16 cases of COVID-19 among kids given dummy shots compared to just three who got vaccinated.
The FDA ultimately assessed more children — 3,100 — who received the kid dosage to conclude it was safe. Youngsters experienced similar or fewer temporary reactions — such as sore arms, fever or achiness — that teens experience.
But the study wasn’t large enough to detect any extremely rare side effects, such as the heart inflammation that occasionally occurs after the second full-strength dose, mostly in young men and teen boys. It’s unclear if younger children getting a smaller dose also will face that rare risk. FDA pledged Friday to keep a close watch.
Some parents are expected to vaccinate their children ahead of family holiday gatherings and the winter cold season.
Laura Cushman of Salt Lake City plans to get her three children — ages 7, 9 and 11 — vaccinated as soon as possible.
“We just want them to get to resume their pre-COVID life a little bit more. And feel safe about it,” she said.
But a recent Kaiser Family Foundation survey suggests most parents won’t rush to get the shots. About 25% of parents polled earlier this month said they would get their children vaccinated “right away.” But the remaining majority of parents were roughly split between those who said they will wait to see how the vaccine performs and those who said they “definitely” won’t have their children vaccinated.
The similarly made Moderna vaccine also is being studied in young children, and both Pfizer and Moderna also are testing shots for babies and preschoolers.