10-year-old’s cholesterol was over 800. Can CRISPR fix the problem?
Verve Therapeutics is considering a half-dozen candidate genes that could be edited with the CRISPR technique in order to sharply reduce a patient’s levels of cholesterol or triglycerides.
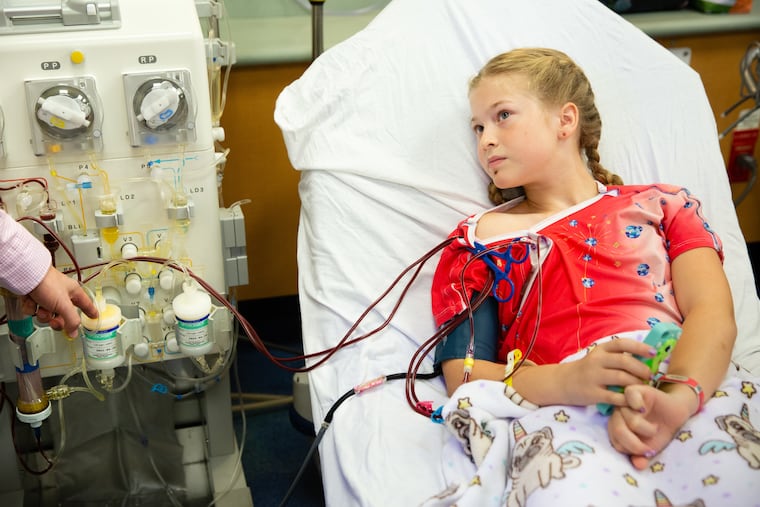
Every other Monday morning, 10-year-old Avery Watts must lie in a hospital bed for three hours to have cholesterol filtered from her blood.
A nurse connects a tube to a port implanted beneath the skin below her right collarbone. Blood travels from there to a whirring machine beside her bed at Nemours/Alfred I. duPont Hospital in Wilmington. A series of pumps and filters extracts a steady trickle of yellowish LDL “bad” cholesterol into a collection bag, then returns the filtered blood to her body through a second tube.
This treatment, called apheresis, is administered to patients with a rare genetic predisposition for ultra-high cholesterol levels. Avery’s levels rise close to 500 by the time she comes in for her twice-monthly session with the machine, which slashes it back below 125.
In May, a biotech start-up announced it was pursuing a one-time treatment that would render such machines obsolete, using a gene-editing technique called CRISPR to lower a patient’s cholesterol levels permanently.
The company, Verve Therapeutics, was cofounded by a trio of prominent researchers with expertise in cardiology and genetics, including the University of Pennsylvania’s Kiran Musunuru. Based in Cambridge, Mass., Verve has secured $58.5 million in Series A financing, led by GV (formerly Google Ventures).
Michelle Watts, Avery’s mother, said she would be interested in the possibility of a one-time treatment for her daughter’s condition, called homozygous familial hypercholesterolemia (FH), which is estimated to strike 1 in 300,000 people. But she said the family would need assurances that any such approach was safe. They already have passed up another treatment option — a liver transplant — meaning that Avery will continue with her regular blood-filtering visits for the foreseeable future.
“We want to be aggressive, but we also want to be careful,” Michelle Watts said (For more information about FH, go to thefhfoundation.org).
What has surprised some in the cardiology community is Verve’s stated goal of someday offering such treatments to a far wider population: those with everyday coronary artery disease. These are patients with higher-than-normal levels of cholesterol not because of any one genetic culprit, as in Avery’s case, but because of a combination of genetic and environmental factors such as poor diet and a sedentary lifestyle.
Musunuru, an associate professor of cardiovascular medicine and genetics at Penn’s Perelman School of Medicine, said he could imagine offering a CRISPR gene-editing treatment to people recovering from a heart attack while they are still in the hospital.
“You don’t have to think about it again,” he said. “They are protected for the rest of their lives.”
If such treatments were shown to reduce the risk of a second heart attack, he said, eventually they could be administered even to people who have never suffered one but are at high risk — a strategy known as primary prevention.
Though heart disease is sometimes described as a Western problem, brought on by a fatty, sugary diet, it is surging in developing countries. By treating it on a population-wide level with a gene-editing technique, physicians could realize the same kind of public-health success that has been achieved with vaccines, Musunuru said.
Years of additional research are needed before such treatments can be offered, but if what Musunuru says comes to pass, the market would be huge.
Heart disease on the rise
Among those who urge caution is Rita F. Redberg, a cardiologist and professor of medicine at the University of California, San Francisco. Cholesterol levels explain only part of a person’s risk for heart disease, and gene-editing treatments would be an expensive alternative when much good can be achieved with exercise and healthy eating, she said.
“It’s very hard to reproduce the benefit of lifestyle or diet with a pill,” she said.
Musunuru agreed that lifestyle modification is an important strategy to ward off heart disease, along with statins — cholesterol-lowering drugs that in many cases are available as inexpensive generics. Yet many patients do not stick with these preventive measures, and heart attacks can occur even in those who do.
“Despite our best efforts, the reality of the situation is that the rate of heart disease is not falling,” he said. “It’s on the rise.”
Other founders of Verve include Sekar Kathiresan and J. Keith Joung, both professors at Harvard Medical School. Kathiresan will serve as chief executive officer, while Joung is strategic adviser and Musunuru is chief scientific adviser.
With research labs housed in the Pennovation incubator facility in West Philadelphia, the team is considering a half-dozen candidate genes that could be edited with the CRISPR technique in order to sharply reduce a patient’s levels of cholesterol or triglycerides, Musunuru said. The specific edits would be based on mutations that are found to occur naturally in a small number of people with ultra-low cholesterol or triglycerides, whom Musunuru referred to as “genetic superheroes.”
A patient would receive a one-time infusion of lipid nanoparticles — little fatty spheres loaded with two kinds of molecular editing “machinery." One component would seek the correct genetic location to be edited, and the other would make the necessary fix. Scientists have developed a variety of these CRISPR editing methods, all loosely inspired by a component of the immune system in bacteria that was discovered barely a decade ago.
Other research teams are pursuing CRISPR-based therapies, including one trial already underway at Penn’s Abramson Cancer Center. So far, researchers there have removed immune-system T-cells from two patients with relapsed cancers, one with multiple myeloma, the other with sarcoma. The cells were edited so they would target the patients’ cancers, then reimplanted in their bodies. Results have not yet been published.
In Verve’s approach, the fatty spheres would travel through the patient’s arteries to the liver, taking advantage of that organ’s ability to metabolize substances in the bloodstream. The editing machinery would then penetrate the nucleus of liver cells and perform the intended edits, Musunuru said.
Mindful of the controversy that arose in 2018 when a Chinese scientist announced he had used CRISPR to edit the genomes of two human embryos, the company has posted this disclaimer on its website: “All of the therapeutics to be developed by Verve involve making edits in adult (somatic) cells, which are not passed down to offspring. We will not edit embryos, sperm cells, or egg cells.”
No mistake
For patients who currently must undergo the blood-filtering apheresis procedure every week or two, it is easy to imagine the potential appeal of a once-and-done treatment.
Only a few dozen U.S. facilities own the filtering devices, so some patients travel long distances for their treatments, said retired cardiologist Samuel S. Gidding, who treated Avery Watts while he was at duPont Hospital. He is now medical director of the nonprofit FH Foundation.
Avery lives in Hagerstown, Md., a three-hour trip from the hospital in Wilmington. She and her mother commonly drive up the day before and stay overnight, then arrive at the hospital at 8 a.m., armed with a bagful of craft supplies to pass the time while her blood is pumped through the machine.
Her high cholesterol was discovered during a regular checkup at age 6. Pediatricians often do not test the cholesterol levels of young children, but Avery’s mother insisted, as she and her husband both had higher-than-normal cholesterol levels.
The result was so high — over 800 — the pediatrician ordered a repeat test. It was no mistake.
It turned out that each of her parents had one copy of a genetic mutation that caused high cholesterol levels. Avery had inherited both. She started the blood-filtering treatments soon after her seventh birthday.
She also takes statins and a second drug, ezetimibe, to get her number down below 500. The biweekly apheresis treatments bring it below 125, but it climbs back to near 500 by the time of the next visit.
Guidelines vary depending on the patient’s age and other risk factors, but generally, it is considered wise to reduce total cholesterol to at least 150 (corresponding to an LDL “bad” cholesterol of about 100).
Lately, there have been signs that Avery’s regimen may not be enough. She felt chest pain and shortness of breath after a cross-country race last fall, and tests revealed that her high cholesterol levels had caused a condition called aortic stenosis — a stiffening of the aortic valve in her heart, Gidding said.
This fall, she will start getting the apheresis treatments every week instead of every two weeks, though she hates to miss school and counts the minutes until she gets home on Monday afternoons.
“We usually get home at 3 p.m. or 3:30,” her mother said during a recent treatment.
“No, 2:30 p.m. or 3,” Avery corrected her.
Because the tubes are connected to the port in her chest, Avery has her hands free for activities, such as scrolling through photos on her smartphone or playing with a container of slime that she made at home. (Favorite experimental ingredient? “Shaving cream!” she said.)
But it is clear the long hours of confinement are a drag. They also are costly. Though the treatments are partly covered by the family’s insurance, they still pay $8,000 a year in out-of-pocket costs, not counting the wear and tear on their car.
For now, Avery’s doctors say this is the way to go, short of a liver transplant. But if the scientists at Verve are successful, someday she may have another choice.