Medical mystery: A non-healing bruise signaled a serious problem
Instead of healing, the bruise got worse and worse, becoming more and more painful and not responding to over-the-counter pain medication.
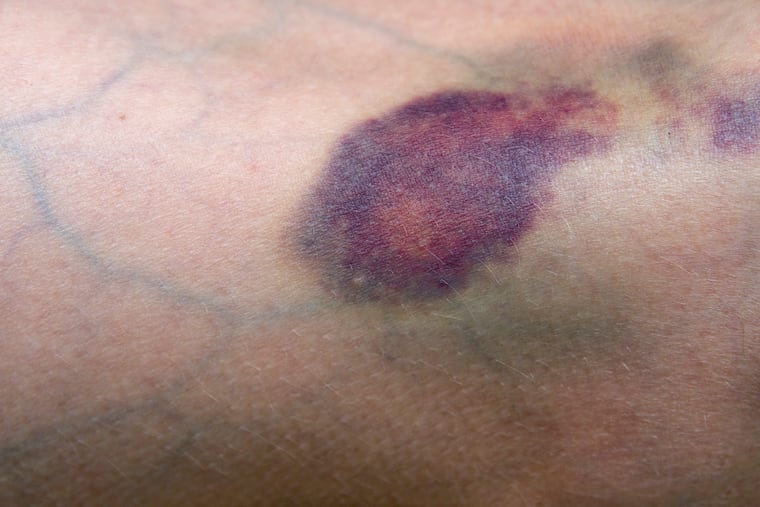
I first met a 62-year-old man in the emergency room while on call for dermatology consults. He said he had a bruise on his leg for months that wouldn’t go away.
Once we began talking, the man, a karate instructor, explained that the bruise had started after a kick from a student. Instead of healing, the bruise got worse and worse, becoming more and more painful and not responding to over-the-counter pain medication.
Unfortunately, he was not an otherwise healthy patient. He had chronic kidney disease, for which he received dialysis as he waited for a kidney transplant.
This combination of a non-healing lesion on the leg and kidney disease immediately made me worry about a terrible condition called calciphylaxis.
Calciphylaxis is a rare disease in which, most frequently in the setting of kidney failure, calcium is deposited throughout the body. The word calciphylaxis, to me, ironically sounds fun, like a wild party of calcium going everywhere. In reality, it’s anything but fun.
» READ MORE: Medical Mystery: Young athlete had pain in his groin for months, but no previous injury
Calcium can be deposited in the skin, essentially turning blood vessels into bones. The calcium deposits then prevent adequate blood flow and cause the formation of painful ulcers and large thick scabs. These ulcers tend to persist and grow despite treatment and become infected with bacteria that can enter the skin and the blood, eventually causing a whole-body infection. As a result, more than half of people with calciphylaxis will die within a year of diagnosis.
We generally think of calcium as a good thing — it’s needed for strong and healthy bones, right? But calcium in excess can be a huge problem. For instance, cardiologists can measure the levels of calcium in heart blood vessels to detect risk for a heart attack.
After evaluating the patient, I performed a skin biopsy to confirm my hunch.
Solution
The biopsy unfortunately confirmed the diagnosis of calciphylaxis. The condition is more likely than not to be fatal, and we don’t know what the best treatment is.
In science experiments, we are taught back in elementary school, you can change only one variable at a time to see whether something works. Otherwise, you can’t make proper attribution of cause and effect. But when patients with problems such as calciphylaxis are staring down a potentially fatal diagnosis, and we don’t have a perfect treatment, we can’t think of them as science experiments. They don’t care about proving that one treatment works. They care about getting better.
As a result, our treatment for calciphylaxis is often what we call “the kitchen sink,” throwing everything we have at the problem at once, because the problem is far worse than any risks of treatment. We can give patients infusions or injections of medicines that bind calcium so it can be excreted (intravenous sodium thiosulfate), medications to dilate the blood vessels (pentoxifylline), and take them off blood thinners that may promote the disease.
When the transplant team heard that my patient had a non-healing wound on his leg caused by calciphylaxis, he was taken off the kidney transplant list because of risk for infection. Talk about a Catch-22: His kidney disease was causing his calciphylaxis, so now he wouldn’t qualify for the one treatment that would fix both his kidney problem and likely his calciphylaxis. With no other good choice, my patient agreed to the kitchen sink approach.
» READ MORE: Medical Mystery: Ongoing knee pain, with no relief from steroid injections
The calcium-binding sodium thiosulfate infusions, given concurrently with dialysis, made my patient nauseated. He didn’t think he could continue to tolerate them. Simultaneously, we tried the same calcium-binding medicine given directly as local injections to avoid the nausea, and they were painful, although better with local anesthesia. He tried his best not to complain, admitting any difficulty only after I questioned him repeatedly.
Our plan of attack seemed ramshackle, but he stuck with it. And with time, the results seemed to justify the approach: His leg wound started to heal, with slow but sure progress at our monthly check-ins. After four months, despite the odds, it had completely healed.
This patient did unusually well with the treatment; many of my patients with calciphylaxis have died from it. I remember another patient, only about 30 years old, whose calciphylaxis left her unable to walk or stand due to the painful wounds. Soon, the ulcers only got worse and worse, leading to infection and death. Every time I make this diagnosis, I can’t help but picture her.
This time, the regimen of many therapies stacked together worked for the patient, and put him back in line for a kidney. But which one did it? Did only one help and the others did nothing? Did some help and others possibly slow it down? Or did all contribute equally? We will never know, but what’s important is that he got better, and hopefully with more time and experience, we can help more patients overcome calciphylaxis.
Jules Lipoff is an assistant professor of dermatology at the University of Pennsylvania Perelman School of Medicine.