These microbiome experts at Rutgers warn of an ‘invisible extinction’ that’s harming human health
Probiotics are big business, but in a new documentary, these scientists say the science of repairing the microbiome remains in its infancy.
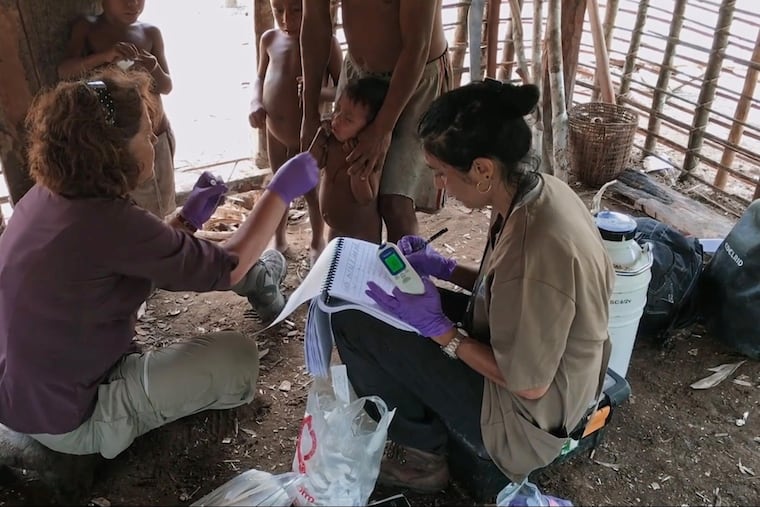
When Maria Gloria Dominguez-Bello travels to the Amazon jungle and tells villagers the reason for her visit, their first response is often laughter.
“Did you come all this way just to see my poop?” they ask.
She did — no humor intended — and she has been doing it for more than 20 years.
She and husband Martin Blaser, both scientists at Rutgers University, are the stars of a new documentary called Invisible Extinction, describing their years of research on how modern diet and medicine are disrupting our internal colonies of bacteria and other microbes — the human microbiome.
The microbiome has been a hot topic for well more than a decade, as they and other researchers continue to identify connections between the loss of “good” bacteria and a variety of human diseases, such as obesity, certain cancers, and autoimmune disorders. Yet the science of how to reverse these problems remains in its infancy.
That’s the message that the Rutgers couple hope to convey in the film, which premiered March 24 at a Copenhagen film festival. They are racing to identify which kinds of bacteria are essential to human health, and how they might be restored through the use of targeted probiotics and other treatments.
The couple think the fecal samples from the Amazon are a big part of finding the solution, as they are teeming with microbes that have yet to be altered by antibiotics or sugary, low-fiber diets, Dominguez-Bello tells filmmakers Steven Lawrence and Sarah Schenck.
“We seek answers in places where the problem hasn’t yet begun,” she says.
No screenings of the documentary have been scheduled yet in the United States, but the filmmakers are on the hunt for a streaming service. More information and a movie trailer can be seen at theinvisibleextinction.com.
‘Giving back bacteria’
In the meantime, the Rutgers scientists are helping to create the Microbiota Vault: a secure, subzero repository to preserve the full richness of the microbiome — including species found in the oral and fecal samples from the Amazon.
A pilot-phase storage facility has already been set up in Switzerland, and it could someday be a source of treatments, Blaser said recently before flying to Denmark for the film premiere.
“One day,” he said, “we will probably be giving back bacteria to children just to restore the ancient organisms that they have lost.”
In their research, the Rutgers couple explore how a variety of modern practices can alter the microbiome, such as diet, the overuse of antibiotics, delivering babies by C-section, and the use of infant formula in lieu of breastfeeding.
The answer, they say, is not to reject drugs, C-sections, and other elements of modern medicine, as all can save lives. The key is to use them only when appropriate, so as to minimize collateral damage to the microbiome.
When antibiotics are overused, for example, not only do the drugs wipe out beneficial bacteria (along with the disease-causing pathogens for which they are designed), they also clear the way for any drug-resistant microbes to take hold.
The overuse of antibiotics is often portrayed as a problem of the developing world, where the drugs are sometimes administered without a prescription, says Blaser, a physician and author of a 2014 book, Missing Microbes, that explores many of the same themes.
But he cautioned that the drugs are overused in developed countries, too. He cited a 2018 study that found Spain and Greece were among the top antibiotics users per capita, and a 2014 study by researchers at Children’s Hospital of Philadelphia, who found that some pediatric practices prescribed twice as many antibiotics as others — a difference that could not be explained by the patients’ medical histories or demographic factors.
The Rutgers researcher, whose lab is in Piscataway, is candid about his family’s own experience with apparent overuse of the drugs. In the film, he speaks with Genia Blaser, his adult daughter from a previous marriage, about her diagnosis with celiac disease. She suffered repeated ear infections as a child and underwent many rounds of antibiotics, as was common practice. Years later, she was treated with powerful antibiotics after contracting a food-borne illness in Peru.
Blaser thinks the drugs contributed to her celiac condition, disrupting her immune system so that it reacts to foods containing gluten.
“To me, that combination of those early childhood antibiotics and those later antibiotics, that’s kind of what led you to this problem,” he tells her. “Which, of course, I feel terrible about.”
Among Blaser’s research specialties is a common bacterium called H. pylori, which causes ulcers. When that connection was proven years ago, some physicians argued that the microbe should be eliminated in everyone. Not a good idea, according to Blaser’s research. These bacteria are present in many people’s microbes with no ill effects, and they appear to have a protective effect against certain cancers.
The flexible biome
Sometimes Blaser and his wife, whose lab is in New Brunswick, conduct studies together, other times independently.
Dominguez-Bello, a native of Caracas, Venezuela, comes at the problem through the lens of urbanization and economic development, studying differences in the microbiome across rural, small-town, and big-city environments in South America.
Once, during a trip to the Amazon, she even studied the impact of diet on her own microbiome.
She and four colleagues had arranged with the locals to stay in their village and eat their food. That meant fruits, vegetables, some fish, and occasional game meat — but nothing like the fatty, juicy varieties on North American grocery shelves.
“It’s so hard,” she said. “It’s like chewing the sole of a shoe.”
After a month on the Amazon diet, all had lost weight. And as Dominguez-Bello predicted, before-and-after fecal samples revealed that their microbiomes had changed, too — though the impact was less dramatic for the five adults than for two children who came on the trip.
Certain types of bacteria became more abundant in the guts of all seven, whereas the children’s microbiomes saw gains not only in abundance but also in diversity. After a month, analysis of the children’s feces revealed types of bacteria that had not been there before.
“We gained on evenness,” Dominguez-Bello said. “They gained richness.”
It was just a small sample, yet the results were consistent with earlier research suggesting that the human microbiome is more plastic — flexible — early in life.
Still, none of the changes was permanent, once the visitors returned to their usual diet and other habits back home.
Restoring one’s microbial diversity is harder than it sounds. One option is a fecal transplant, a technique that has helped some patients battle an infection called C. difficile. But research is needed to develop a more targeted approach for treating other conditions, Blaser said.
Another option is the oral supplements called probiotics. Yet more work is needed there, too, as many current products are more about marketing than scientific substance, Blaser said.
So the research continues, with a strong emphasis on communication.
In the Amazon, for example, Dominguez-Bello takes pains to teach villagers about her mission, bringing explanatory posters and microscopes so they can see the microbes in question. She also works with local scientists to set up labs and microbe collection facilities.
“The times that rich countries went to poor countries and extracted things, those days are over,” she said. “We have to train them and educate and them and empower them.”
The result, she and Blaser hope, will be better for the long-term health of all.