This new ovarian cancer treatment could improve survival rates | 5 Questions
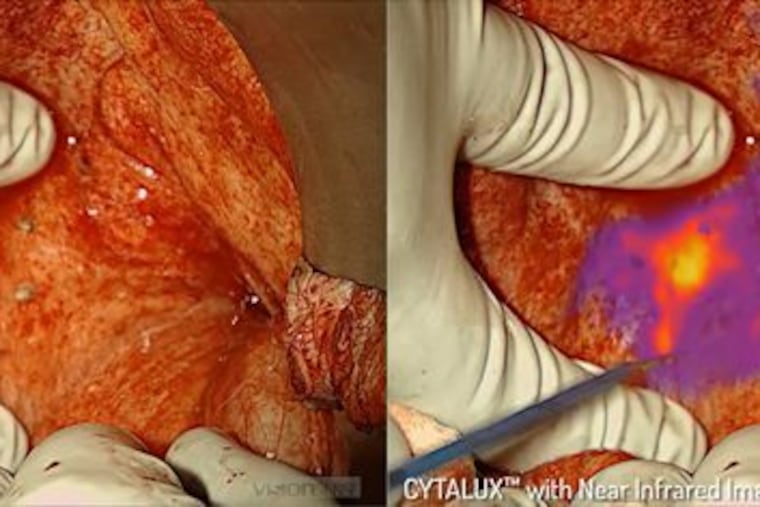
In November, the U.S. Food and Drug Administration approved an agent called Cytalux, which binds to cancer cells and then, during surgery, glows with near-infrared fluorescence imaging.
Every year in the U.S., 22,000 to 24,000 women are diagnosed with ovarian cancer.
Their survival depends, in part, on surgical procedures that aim to remove all of the cancerous tissue. In about half of ovarian cancer patients, however, the disease returns after their initial treatments.
But in November, the U.S. Food and Drug Administration approved a new method that improves surgical outcomes and might change the odds for women with ovarian cancer. It involves use of an agent called Cytalux, which binds to cancer cells and then, during surgery, glows with near-infrared fluorescence imaging.
One of the largest trial sites for Cytalux was the Center for Precision Surgery in the Abramson Cancer Center at the University of Pennsylvania, in partnership with On Target Laboratories, an Indiana biotechnology company that developed the new procedure and is working on similar treatments for other cancers.
We spoke recently to the principal investigator for Phase 2 and Phrase 3 trials at Penn, Janos L. Tanyi, who holds both a medical degree and a doctorate and is an associate professor of obstetrics and gynecology in the Perelman School of Medicine.
More than a decade ago, researchers began searching for a better way to detect ovarian cancer cells and tumors. What were the limitations you were trying to overcome?
Generally, ovarian cancer has no symptoms in early stages. And there is no screening test like the ones for breast cancer and cervical cancer. So by the time a diagnosis is made, many women are at an advanced stage of the disease, and the cancer has often spread to other organs in the abdomen. The tumors need to be removed surgically.
The standard of care for ovarian cancer is a combination of surgery and chemotherapy. Surgery can happen first, with chemotherapy afterward, or the patient can start chemotherapy first, with surgery somewhere in the middle.
The goal is to remove all of the cancer, but this can be difficult. For decades, surgeons have had to depend on their eyes to visually detect cancer and depend on palpation to feel tumors in the abdominal cavity. The term is cytoreduction, or debulking, but it’s basically removing as much of the cancer as you can find.
But one study showed that even when we believed we had removed all the disease, sometimes tumors or cancer cells still stayed behind. That study found that among patients who had undergone even optimal cytoreduction, post-operative imaging 30 days after the surgery showed that 40% had measurable disease.
Later, in our Phase 3 studies for Cytalux, we found that 27% of women undergoing surgery for ovarian cancer had at least one cancerous lesion, beyond those detected by palpation or under normal light, that was detected using the new procedure. This means that without it, 27% of patients would walk out of the hospital with the belief that all cancer was removed, while in fact some cancer stayed behind.
How does Cytalux work?
The agent is administered intravenously at least one to three hours before the surgery. It basically sticks to the folate receptors that are overexpressed – more numerous, one could say – on the surface of ovarian cancer cells, and it accumulates in the cells. Because of this, when we use an imaging camera with near-infrared fluorescent light, the tumor cells glow. It makes even a hidden tumor very visible.
So Cytalux improves our chances of removing all the cancerous lesions. The technology also can help us spare healthy tissue because we only have to remove the portion that lights up. Overall, this technology helps us to provide more precise care and better care for our patients.
This was a very novel application of imaging technology. It had never been done before. Others are in development, but this kind of intra-operative molecular imaging has never gone this far – through FDA approval – for any other cancer.
Why was Penn one of the largest trial sites?
At our Precision Surgery Center, we are testing multiple agents, not just this one. Since the center’s founding in 2015, our researchers and those from affiliated labs have developed several other targeted imaging technologies. We have had a high level of experience using these imaging agents.
Why is this a game-changer for patients with ovarian cancer?
Because it extends lives. Over the past 10 to 15 years, there have been multiple studies that have shown that better debulking leads to longer survival. Those patients who have all the visible cancerous lesions removed have the best survival rates, so optimal and complete resection of disease during cytoreductive surgery remain independent prognostic factors for overall survival.
Here’s an example: With one of my Phase 2 trial patients here at Penn, I knew ahead of surgery that there were two small nodules of ovarian cancer. But during the surgical procedure, by using this new methodology, I discovered that about two inches away from the known tumors was a small area that was also cancerous. Even knowing where it was, I palpated it and couldn’t feel it. I looked at it under normal light and didn’t see anything. But with the new agent and under the near-infrared fluorescent light, it kept lighting up. Indeed, it was an extra tumor lesion which would have been left behind otherwise.
I removed it, and for the next two years, the patient had no evidence of disease. If I had left that nodule behind, within three months she could have been full of cancer again. This extended her life and gave her a better quality of life.
Could this potentially be adapted for use with other cancers?
Absolutely. These agents target folate receptors, which as I’ve said are overexpressed on the surface of ovarian cancer cells. But other cancer types also have folate receptors. I strongly believe that these can be targeted with the same agent.
There is the potential for a much bigger application for this agent in addition to what the FDA recently approved.
Right now, Phase 3 trials for treating lung cancer with Cytalux are going on here at Penn. In other trials, the indications keep increasing. The diagnoses that this agent might be used for keep increasing.
This will help many, many patients.