A half-million dollars a year for one drug
In 2010, Forbes declared Alexion Pharmaceuticals' Soliris the world's "single most expensive drug," at nearly $410,000 a year. It seems to have gone up since.
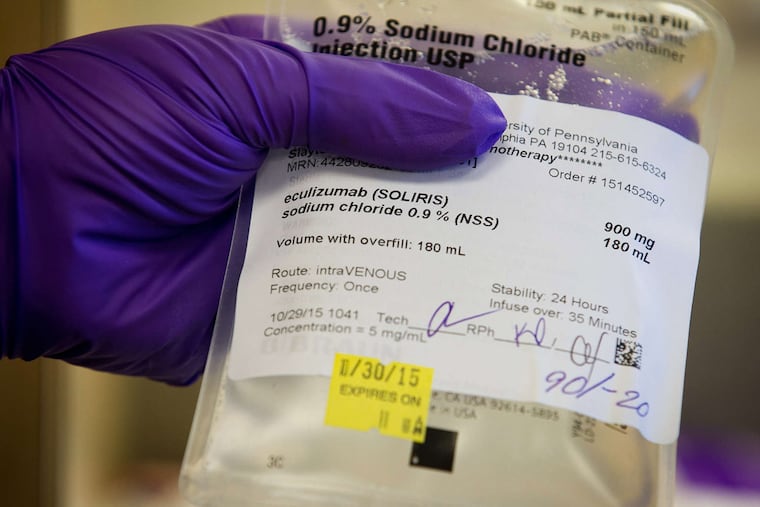
In 2010, Forbes declared Alexion Pharmaceuticals' Soliris the world's "single most expensive drug," at nearly $410,000 a year.
It seems to have gone up since.
An organization representing Canadian provinces announced two weeks ago that it had called off negotiations with the drugmaker over its $500,000 asking price. A watchdog agency in the United Kingdom said the company was seeking $700,000 there.
In the United States, hospitals buy the drug, which is infused like chemotherapy, and they bill insurance companies directly. With infusion and lab costs added in, charges for treatment every other week can top $1 million annually. Insurers cover perhaps two-thirds of that amount (accepted as payment in full).
Most drugs have competition to help drive down prices. Medicines for rare diseases often do not.
Alexion's stock price has risen nearly 1,500 percent since the first approval of Soliris, which remains its only marketed drug, in 2007. The monoclonal antibody, whose chemical name is eculizumab, is now approved to treat two rare diseases but not systemic Degos.
Alexion initially agreed to provide it on a free "compassionate use" basis to the first patients who were treated "off label" for Degos disease. The Cheshire, Conn., biotech firm later insisted insurers take over.
It considers providing its products for patients in clinical trials, company spokeswoman Kim Diamond said in an email. She added: "We are currently not studying the use of eculizumab for the treatment of Degos disease."
Now that a handful of patients on the drug are living with a disease that is otherwise fatal, a half-dozen insurers are paying for it on a case-by-case basis.
In general, coverage of an experimental treatment "is not based on cost. It is based on whether there is proven benefit," said Ginny Calega, a vice president of Highmark Blue Shield, the insurer for Theresa Slayton, a nurse near Scranton who has been on eculizumab for systemic Degos for four months.
Calega would not discuss how much of the billed charges Highmark reimburses or the implications of paying hundreds of thousands of dollars for a single drug.
"If you squeeze the balloon somewhere, it has to come out someplace else," she said. "It's why the cost of pharmaceuticals is such a hot topic right now."