Easing a lifetime of pain: Philly doc develops one-shot treatment for inherited bleeding disorder
At a Home Depot not long ago, Jay Konduros was hefting a 40-pound box of shelving into his cart when it suddenly slipped — and bam!
The blood welled up from a gash in his shin, and his mind flashed back to a lifetime of caution and pain. That minor mishap with a snow shovel that put him out of commission for weeks, his leg so swollen he could not see his knee.
The many days he told friends he could not come out to play, but didn't want to say why. The times his mother rushed him to the hospital, carrying him because he couldn't walk.
But then, staring at his gashed shin at Home Depot, he thought this time might be different.
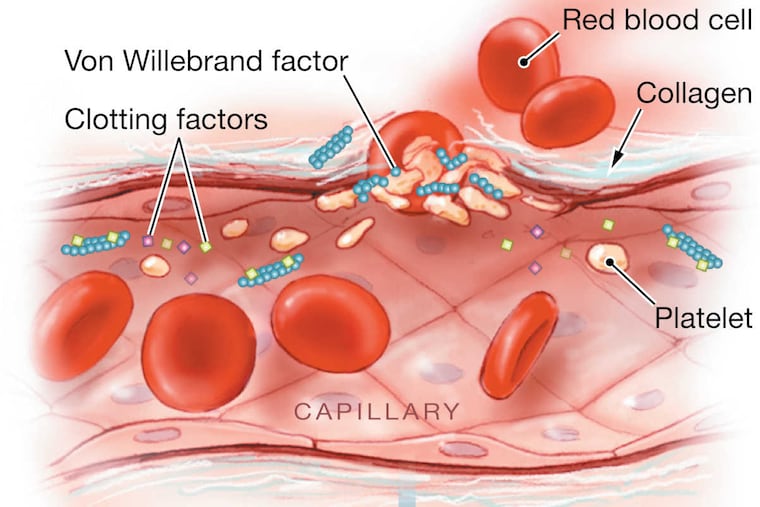
Konduros was born with hemophilia, the blood-clotting disorder.
Then last year, the 52-year-old was among the first in the world to receive a one-time, experimental treatment: a dose of genetically engineered particles that would enable his liver to make enough clotting factor on its own.
If he was lucky, this one-shot treatment -- developed by Spark Therapeutics, a biotech start-up spun off from Children's Hospital of Philadelphia -- would ward off his disease for what could be many years.
The Home Depot accident, near his home outside Toronto, represented an unplanned test of the treatment. In the hours and days after the heavy box slammed into his leg, Konduros waited and watched.
There was no bruising. No swelling. No debilitating pain.
"You kind of smile and think, 'OK, that's abnormal,' " he said.
A genetic recipe
Katherine A. High's first extended experience treating patients with hemophilia came during an especially dark period for the disease.
The advent of donated clotting factors had revolutionized treatment for a condition that once meant a shortened, painful life. But in the 1980s, scientists learned that HIV could be transmitted through these donated blood proteins. As many as half of the patients in the United States -- the condition affects mostly males -- would become infected with HIV. Some also contracted hepatitis C, another serious blood-borne illness.
High, a physician then in her first faculty job at the University of North Carolina at Chapel Hill, wondered if the answer lay in the young field of genetics.
"I desperately wished that we had a better treatment," she said in a recent interview.
In 1989, long before the days of high-speed gene-sequencing machines, she and colleagues announced a breakthrough. They pinpointed the mutation that caused a form of the disease, called hemophilia B, in dogs.
In the 1990s, by then at Children's Hospital, High pursued the idea of treating hemophilia with gene therapy: infusing patients with "vector" particles that carried the genetic recipe for clotting factors they could not make on their own.
High and her colleagues got the treatment to work first in mice, then in dogs. Then in 2006, they announced it had worked in humans, but only temporarily.
That was because the vectors carrying the recipe were made from viruses, which had provoked a response from the patients' immune system.
Viruses are an ideal tool in the field of gene therapy, as they are equipped with the natural ability to invade a human cell and deliver DNA. In the case of the hemophilia patients, the viral vector was loaded with the DNA recipe to make clotting factor. Also key: It was a type of virus that did not cause human disease.
Still, the outer shell of the particles triggered an immune response. Within weeks of treatment, the patients' bodies destroyed the enhanced liver cells, and the benefits of the treatment wore off.
High's team, and others, went back to work. In 2013, she decided the best pathway to success was through the private sector.
With Children's Hospital investing $50 million, High cofounded a biotech start-up called Spark Therapeutics.
Given its aim to produce one-shot treatments for hereditary diseases, the company chose a fitting ticker symbol on the NASDAQ stock exchange: ONCE.
Glad to be in Canada
By the time Jay Konduros was an adult, the standard treatments for hemophilia had made great strides. Careful screening and purification methods all but eliminated the risk of contracting disease from donated clotting factors.
Then came the development of synthetic clotting factor, which was judged to be even safer. And unlike the early days, he could administer the treatment at home. In his case, that was clotting factor IX, the protein that is deficient in people with hemophilia B.
Konduros' doctor told him it cost $30,000 (about $22,500 in the U.S., at current exchange rates) for enough of the precious fluid to treat a typical injury, a cost picked up by Canada's national health service.
"Which made me kind of gulp a bit," he said. "You do think, 'Damn, I'm glad I live in Canada.'"
Depending on the severity of an injury, he might get away with one or two treatments. But if he waited too long, or misjudged the amount, it meant trouble.
When shoveling snow two years ago, the blade caught on the ground and the handle rotated sideways, jamming hard into his thigh. He took two treatments at home, yet ended up in the hospital for 10 days, and had to walk with a cane for months.
In November 2015, Konduros' doctor told him about High and the gene therapy at Spark.
In the latest version of the treatment, the vector was programmed with the DNA of a super-clotter -- a patient in Padua, Italy, whose clotting activity levels were four to eight times normal.
That meant patients could make do with just one-fourth the dose of the viral particles, greatly reducing the risk of an immune reaction.
Konduros came to Philadelphia in June. Lindsey George, a hematologist at Children's Hospital, administered the infusion, known as SPK-9001. Trillions of the special particles traveled through a tube into his arm, destined for his liver.
Then he waited.
The cost
Konduros is one of 10 patients to get the treatment so far. Spark has announced results for nine, all of whose ability to make clotting factor has soared. Two patients experienced an immune reaction and their factor activity levels, after rising, started to decline. But their levels stabilized after they were given steroids, High says.
So far, physicians not involved with the study are impressed.
"It's pretty exciting news," said Jason A. Taylor, associate director of the Hemophilia Center at Oregon Health & Science University. "We're still not exactly sure what's going to happen in the long term."
What should this therapy cost? High won't say, but some analysts have said they expect the company to aim for a treatment price in the range of $1 million, an eye-popping cost even in an era of high drug prices. Yet this is potentially a one-time fix for a disease that can cost many millions to treat over a lifetime. Some in the gene-therapy field have discussed a pay-by-installment model.
In July, the month after Konduros got his infusion, the U.S. Food and Drug Administration granted "breakthrough" status to the therapy, allowing for an expedited review process.
Pharmaceutical giant Pfizer has bought the right to sell the therapy, if it is approved, and has paid Spark $50 million to date. The start-up is eligible for up to $230 million more plus royalties.
High, the company's president and chief scientific officer, is optimistic.
"For all of these people, it's a little bit like they come in, they get the infusion, they walk out, and they leave their hemophilia behind," she said. "How long will it last? I don't know. Were they very brave to volunteer? Absolutely."
She refuses to call it a cure, since the therapy is too new to be certain how long the effects will last. In dogs who got the treatment, the improvement has lasted more than a decade so far.
Konduros, who runs a bakery in Kitchener, Ontario, calls it "The Philadelphia FIX," playing on the abbreviation for factor IX.
He is speaking out about it because already he sees a future when the disease is no big deal. He equates it to the huge advances made with vaccines that have turned childhood scourges like polio into distant memories.
"They'll say, 'What's hemophilia? Apparently, people in the past had it,' " Konduros said. "They won't know what they're missing."