Emphysema patients get new option as FDA approves lung valves studied at Temple
The FDA has approved lung-shrinking valves as a novel treatment for advanced emphysema. The valves were developed with the help of Temple University researchers.
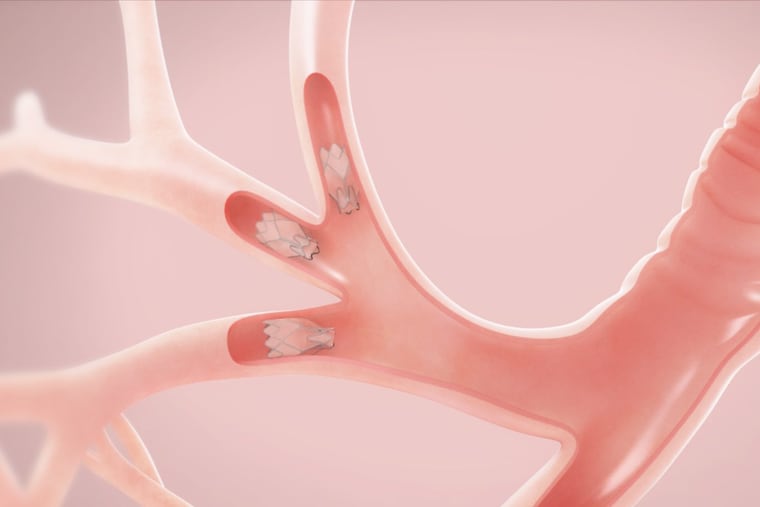
The U.S. Food and Drug Administration on Friday approved a novel treatment for severe emphysema: one-way air valves that are implanted in the lungs to redirect air.
The Zephyr endobronchial valves, made by California-based Pulmonx, were shown to improve lung function, exercise capacity, and breathlessness in an international study led by Temple University.
In suitable patients, two to eight valves are strategically placed in tiny airways using a scope that is threaded through the mouth into the lungs. The pencil eraser-size valves expand and seal the airways, shrinking diseased areas of the lungs so healthier regions can expand and function better.
This compensates for the fundamental problem of late-stage emphysema: As tiny air sacs are destroyed, air gets trapped in diseased regions, causing overinflation that leaves sufferers literally stuck holding more and more of their breath.
For patients who don't get relief from standard inhalers, steroids, and oxygen, the only other options are risky surgery, to cut away diseased parts of the lungs, or a lung transplant.
"This novel device is a less invasive treatment that expands the options available to patients." FDA official Tina Kiang said.
More than 3 million Americans have been diagnosed with emphysema, a progressive, incurable disease usually caused by smoking. Gerard Criner, the Temple pulmonologist who led the international trial, estimated that a few hundred thousand patients might benefit from the valves.
"This marks an important step forward to have a minimally invasive therapy to treat patients with advanced emphysema and severe hyperinflation," he said.
Pulmonx provides a pulmonary assessment system, called Chartis, that is used to identify patients most likely to benefit from the valves and the best placement of the devices.