Rare cancer reignites debate over breast implants' safety
The rare lymphoma has been tied to saline and silicone gel implants, both for enlargement and for reconstruction after breast cancer. However rare, the significance of the mysterious cancer is huge.
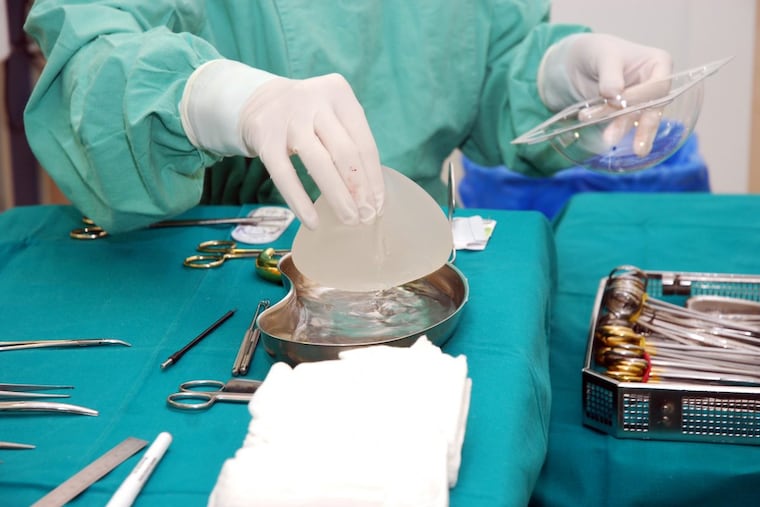
When Kathy Arreola decided to enlarge her breasts in 2000, silicone gel-filled implants were restricted by the government because of safety questions.
She had no qualms about the remaining option, silicone pouches filled with saline.
"I thought, 'These are safe. These are not going to be a problem,'" said Arreola, 54, who lives in Southern California. "I was really happy with them."
That changed abruptly in late 2014. She awoke one morning to a "stabbing pain" in her left breast, which had ballooned because of swelling around the implant.
She was found to have a new type of lymphoma – a cancer of the immune system – caused by implants. Called breast implant-associated anaplastic large-cell lymphoma, the malignancy is distinct from breast cancer and very rare. The U.S. Food and Drug Administration, which first warned of a link in 2011, said in March that it had reports of 359 cases worldwide, including nine deaths.
The lymphoma has been tied both to saline and gel implants, both for enlargement and for reconstruction after breast cancer. Nearly all patients, including Arreola, had textured rather than smooth implants. The roughness may trigger chronic inflammation.
However rare the mysterious cancer, its significance is huge.
For the first time since implants were introduced in the 1960s, medical authorities, including the World Health Organization, agree that the prosthetics give rise to a disease in susceptible women.
Some experts believe that consensus validates the controversial theory that implants can trigger debilitating autoimmune illnesses such as lupus, scleroderma, and fibromyalgia — as legions of women have contended since the 1990s.
"To me, that has to be evaluated again," said Roberto Miranda, a pathologist at MD Anderson Cancer Center in Texas, which has treated about 40 women with implant-related lymphoma.
A mild cancer can turn deadly
The first recognized case of implant-related lymphoma was in California in 1997. By 2010, with a few dozen more case reports, Miranda and some other researchers suggested it was a new disease entity: a rare blood cell malignancy, with even rarer genetic hallmarks, that developed around breast implants and tended to stay there.
The National Comprehensive Cancer Network, based in Fort Washington, issued diagnosis and treatment guidelines late last year. Because the lymphoma is typically not aggressive, it can usually be treated by removing the implant, fluid, and the capsule of scar tissue that naturally forms around the device.
But the cancer can become life-threatening, as Terri McGregor, 52, of North Bay, Ontario, can attest.
"I had no symptoms," the former paving company owner said recently.
None, that is, until days after her first mammogram at age 50 in 2015. The results were normal, but she felt lumps under an armpit. Her doctor assumed the mammogram had ruptured her six-year-old textured gel implants. Ultimately, an MRI and biopsy revealed lymphoma that had spread to her bowel and lymph nodes.
After two chemotherapy regimens failed, doctors estimated she had six months left to live. A newer, targeted Hodgkin's lymphoma drug rescued her, but she still had to undergo a risky stem cell transplant and chest radiation.
The ordeal has turned her into an activist. She co-founded a Facebook group for implant lymphoma patients, made a YouTube video, and recently met with the FDA about improving patient information.
Many missed diagnoses
Worldwide, an estimated 10 million women have implants, including about 4 million in the United States.
In 2011, with reports of 34 cases of the lymphoma, the FDA said establishing a causal link to implants would be almost impossible because a definitive study would have to follow "hundreds of thousands of women for more than a decade."
One implant maker, Allergan, said in a news release, "A woman is more likely to be struck by lightning than get this condition."
But now the FDA webpage says Australian researchers estimate the lymphoma risk could be as high as 1 in 1,000 women with implants.
At the FDA's direction, Allergan and the other big maker, Mentor, have updated package labeling to include the lymphoma. Both companies say they support gathering more data. But a new case registry called PROFILE, created by the FDA and plastic surgeons' groups, declined to share its latest numbers.
The Facebook group now has 60 members with the lymphoma, six times more than when it was created a year ago, McGregor said.
Despite publicity, awareness among doctors remains low, experts agree. The classic symptom of the lymphoma, swelling around an implant, may be mistaken for infection or injury.
"The diagnosis is quite often missed," said Jan Willem Cohen Tervaert, an immunologist at Maastricht University Medical Center in the Netherlands, which has treated about 40 cases of implant lymphoma — including in a transgender woman. "And many implants that are removed are not sent to pathology" to test for the cancer.
Arreola spent more than eight months chasing a diagnosis.
"But I'm one of the lucky ones," she said, "because I was sent to the right people, even though it took a while."
She was also lucky that her health insurance paid for her removal surgery. Cosmetic implants are not covered by insurance, so complication costs are usually excluded.
"Some women in our Facebook group are having a hard time getting insurance approval" for treatment, she said.
How implants might promote diseases
Textured implants, which are treated with chemicals that etch the pouch surface, were introduced in the early 1990s – about five years before the first report of implant lymphoma.
Texturing keeps the device firmly in place because scar tissue grows into the rough surface. This also reduces painful tightening of the scar capsule, called capsular contracture, which is a major reason for implant removal or reoperation.
Steve Copit, chief of plastic surgery at Thomas Jefferson University Hospital, said he now discusses the lymphoma risk with patients.
"But I still use textured implants," he said. "I think it's so rare that the benefits in some patients far outweigh the risks."
How might textured implants promote cancer?
One theory involves the normally harmless bacteria that live in the human body. U.S. and Australian researchers found that the "biofilm" on implants removed from capsular contracture patients was different from the biofilm on 26 implants from lymphoma patients, pointing to "a possible infectious contributing cause" for the lymphoma.
Most researchers believe the culprit is the implant itself, perhaps because of chemicals in the manufacturing.
Both theories are built on the fact that when tissues are injured by foreign invaders, the damaged cells cause blood vessels to leak fluid, producing swelling. Persistent inflammation activates immune cells, including T lymphocytes – the blood cells that multiply and turn malignant in implant lymphoma.
A number of inflammatory diseases are known to cause lymphomas and other cancers, including Sjogren's syndrome, an autoimmune disease that attacks moisture-secreting glands throughout the body; Hashimoto's thyroiditis, which attacks the thyroid gland; and celiac and Crohn's disease, which attack the digestive system.
There are also a handful of reports of lymphomas arising in the tissue around implanted silicone-containing heart devices.
Beyond lymphoma
Published reports as far back as 1964 describe women getting implants and then developing diverse and enigmatic health problems. The illness has been given various names, including siliconosis and silicone incompatibility syndrome.
Seven years ago, Israeli autoimmunity researcher Yehuda Schoenfeld offered a new term: Autoimmune/autoinflammatory syndrome induced by adjuvants, or ASIA. Adjuvants are substances that boost the immune response.
"We believe that the immune response to silicone…includes a systemic component," he and colleagues wrote in 2011 in the Journal of Autoimmunity. "The systemic response…includes a constellation of symptoms: diffuse pain, morning stiffness, fatigue, impaired cognition, depression, headache, dry eyes, dry mouth, …swollen lymph nodes, and unexplained fever."
Cohen Tervaert, the Dutch researcher, subscribes to this theory, even though he acknowledges that most population studies have not found an increased risk of autoimmune diseases in women with implants. In recent years, his medical center has treated more than 200 implant patients with ASIA. Most got better after implant removal — another echo of a pattern seen for decades.
"Despite changes in the principal constituents of the silicone implants during the past 50 years, silicone remained an adjuvant that…may be a chronic stimulus to the immune system resulting in similar clinical manifestations as 50 years ago," Cohen Tervaert wrote in a journal article this year.
After women get rid of their implants and scar capsule, the cosmetic results can be wretched, so many surgeons suggest replacing textured implants with smooth ones.
Arreola declined.
"I thought, 'Are you guys crazy?' This is a foreign body," she said. "If my body reacted this way once, it will again."
Why the science is still murky on breast implant safety
The U.S. Food and Drug Administration lifted 14-year-old restrictions on sales of silicone gel breast implants in 2006, though it still was unclear whether the devices could make some women chronically ill.
That question remains unsettled, because no study has ever followed enough women for enough years to tease out the risks of rare diseases.
That's why the FDA imposed a big condition when it approved silicone gel breast implants 11 years ago: Allergan (then Inamed) and Mentor Corp. each had to follow 40,000 women with gel implants and smaller groups with saline implants for a decade. That would be sufficient to detect increases in connective tissue diseases, neurological disorders, certain cancers, even suicide.
Although the companies reached the enrollment goals, the studies quickly fell apart. Allergan said that at two years, it had lost track of 40 percent of women with gel implants and more than half with saline. Mentor's scorecard at three years was worse; almost 80 percent of gel and 90 percent of saline participants were "lost to follow-up."
In early 2011, soon after the FDA announced that implants might be linked to a rare lymphoma, consumer advocates and U.S. Rep. Rosa DeLauro (D., Conn.) called on the agency to hold a public advisory committee meeting to discuss the studies.
"These statistics would suggest a lack of commitment to these studies by both companies," DeLauro wrote in a letter. "Have the companies been warned that FDA approval could be rescinded if the studies are not properly conducted?"
At the August 2011 FDA meeting, adviser Jason Connor, a statistician who designs clinical trials, pressed that question, a transcript shows.
"Has the FDA ever said your post-approval study is so awful, we're removing your product from the market?" Connor asked. "You know, what is their honest-to-God incentive to do this well?"
FDA official Danica Marinac-Dabic answered that the FDA could rescind approval, but had never done so.
Several women at the meeting testified that they were thrown out of the implant studies for reporting problems.
One of them, Susan Appelt of Mount Juliet, Tenn., is now 61. In a recent interview, she said she was "not anti-implants," but that after she switched from saline to silicone gel, she suffered joint pain, dizziness, heart palpitations, vision problems, and muscle spasms. She finally paid to replace her $6,000 gel implants with saline, began feeling better, and was dropped from the study by her Vanderbilt University plastic surgeon.
"I emailed, wrote, and called Allergan. I told them about my problems. No one ever responded," Appelt said.
Company officials, for their part, stressed that smaller studies supported the approval of gel implants.
After that 2011 meeting, the FDA commissioned Tufts Medical Center researchers to review all available studies of health outcomes in women with gel implants. But the review was funded by the Plastic Surgery Foundation with money from implant makers, hardly neutral sources.
Previous comprehensive reviews of the implant medical literature had been published, the best known being the Institute of Medicine's in 1999. These analyses had concluded that studies show no significant links to rare diseases, but are too limited or flawed to settle the matter.
Tufts' assessment, published last year in Annals of Internal Medicine, reached the same conclusion, although it found "suggestive evidence" that implants increase rheumatoid arthritis, Sjögren's syndrome, and Raynaud syndrome.
"Therefore, in 2015, the FDA notified breast implant makers that they are no longer required to conduct studies to" look for associations, an FDA representative said in a recent email. The agency continues "to monitor for these adverse events" using methods such as voluntary reports from doctors and patients, the email said.
The demise of the manufacturers' large studies — noted in fine print in an FDA online database— came as news to Connor.
"I think it would have been fair for the FDA to publicly announce that the studies that were part of approval have essentially ended without the implant makers fulfilling their obligation," he said. "It was very clear to me that the makers never tried."